How do you analyze a UV-Vis graph?
1) Step 1: Identify the number of peaks appearing in the UV-VIS spectrum. Figure 5 shows several peaks indicating the presence of an excited electron. The easier the electrons are excited, the greater the wavelength that is absorbed, the more electrons are excited, the higher the absorbance.
How do you read absorbance spectrum?
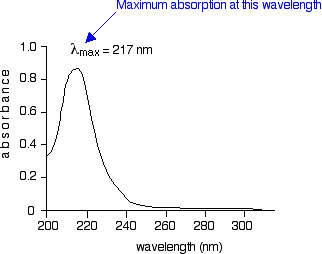
The higher the value, the more of a particular wavelength is being absorbed. You will see that absorption peaks at a value of 217 nm. This is in the ultra-violet and so there would be no visible sign of any light being absorbed - buta-1,3-diene is colourless. You read the symbol on the graph as "lambda-max".
What does UV-Vis spectra tell you?
UV-Vis Spectroscopy (or Spectrophotometry) is a quantitative technique used to measure how much a chemical substance absorbs light. This is done by measuring the intensity of light that passes through a sample with respect to the intensity of light through a reference sample or blank.
What important information can you gain from a UV-Vis spectrum?
UV-vis spectroscopic data can give qualitative and quantitative information of a given compound or molecule. Irrespective of whether quantitative or qualitative information is required it is important to use a reference cell to zero the instrument for the solvent the compound is in.
How do you read a color spectrum?
4:5411:11Visible Light Spectrum Explained - Wavelength Range / Color Chart ...YouTubeStart of suggested clipEnd of suggested clipGreen light ranges from 500 to about 570.. And yellow is very small it's 570 to 590. The range isMoreGreen light ranges from 500 to about 570.. And yellow is very small it's 570 to 590. The range is limited orange goes up from like 590 to 620. So red is from 620 to 700.
How do you tell the color from the absorbance spectrum?
If wavelengths of light from a certain region of the spectrum are absorbed by a material, then the materials will appear to be the complementary color Thus, for instance, if violet light with wavelength of 400nm is absorbed, the material will look yellow. If the material absorbs blue you will see the color orange.
Why are the peaks in UV spectrum broad?
In UV-Visible spectra Bonds will be in constant vibration, this variation will absorb nearby energies i.e, ΔE , for this reason UV peaks are broader. Spectrum is broaden by spontaneous emission.
What is the wavelength range of UV spectrum of light?
100-400 nmThe UV region covers the wavelength range 100-400 nm and is divided into three bands: UVA (315-400 nm) UVB (280-315 nm) UVC (100-280 nm).
What is lambda max in UV-Vis?
Lambda max (λmax): The wavelength at which a substance has its strongest photon absorption (highest point along the spectrum's y-axis). This ultraviolet-visible spectrum for lycopene has λmax = 471 nm.
Why is UV-Vis spectroscopy important?
UV-VIS spectroscopy, like FTIR, is a technique which is useful in the identification of pure drug compounds. Many molecules contain chromophores which will absorb specific wavelengths of ultra violet or visible light.
How do you read the results of A spectrophotometer?
The higher the amount of absorbance means less light is being transmitted, which results in a higher output reading. For example, if 50% of the light is transmitted (T=0.5), then A = 0.3. Likewise, if only 10% of the light is transmitted (T=0.1), then A = 1. Absorbance has also been called optical density (or O.D.).
What does high absorbance mean in Spectrophotometry?
When you get very high absorbance (>1.5), it means that most of the light are absorbed by the sample and only small amount of the light detected by detector.
How does absorbance relate to wavelength?
Molecules will have a certain range of absorbance with a peak at a certain point. A wavelength longer than the peak absorbance and shorter than the peak absorbance will result in more light being recorded by the detector.
How do you measure absorbance?
Absorbance is measured using a spectrophotometer or microplate reader, which is an instrument that shines light of a specified wavelength through a sample and measures the amount of light that the sample absorbs.