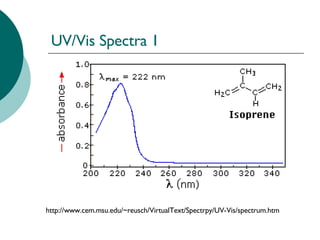
Interpretation of this UV-Visible spectra . First find out wave length of maximum absorption.It looks above 380 nm this means that compd. absorbs in the near UV region. Usually conjugated compds. show absorption above 240 nm. As absorption is above 38o nm which indicates that it is congugated compd. conaining double bonds.
What is the UV Vis spectrum?
UV-vis spectroscopy is a cost-effective, simple, versatile, non-destructive, analytical technique suitable for a large spectrum of organic compounds and some inorganic species. As a function of wavelength, UV-vis spectrophotometers measure the absorption or transmission of light that passes through a medium. In order to classify and measure the ...
What are some uses of UV/Vis spectroscopy?
13.21.1.1: Some Uses of UV/Vis Spectroscopy
- Electronic transitions. Let's take as our first example the simple case of molecular hydrogen, H 2. ...
- Looking at UV-vis spectra. We have been talking in general terms about how molecules absorb UV and visible light - now let's look at some actual examples of data from ...
- Applications of UV spectroscopy in organic and biological chemistry
What is maximum absorbance wavelength?
What is maximum absorbance wavelength? The absorption is highest at around 510 nm (the wavelength at which absorption reaches its peak is called absorption maximum wavelength). How is the UV absorption maximum of paracetamol determined? A UV absorption maximum was determined by scanning 10µg/ml solution of paracetamol in phosphate buffer 6.8, in between 200-400 nm by using UV-visible spectrophotometer.
What is ultraviolet visible spectroscopy?
Ultraviolet–visible spectroscopy or ultraviolet–visible spectrophotometry (UV–Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. This means it uses light in the visible and adjacent ranges.
What does UV-Vis spectra tell you?
UV-Vis Spectroscopy (or Spectrophotometry) is a quantitative technique used to measure how much a chemical substance absorbs light. This is done by measuring the intensity of light that passes through a sample with respect to the intensity of light through a reference sample or blank.
How do you explain UV-Vis graph?
0:292:11How to Interpret a UV Vis Graph - YouTubeYouTubeStart of suggested clipEnd of suggested clipDifferent colors are seen. So at about 400 nanometers blue or violet light is absorbed and on theMoreDifferent colors are seen. So at about 400 nanometers blue or violet light is absorbed and on the other end of the spectrum at 700 nanometers red light is absorbs. And every other color is in between.
How do you interpret an absorption spectrum?
The higher the value, the more of a particular wavelength is being absorbed. You will see that absorption peaks at a value of 217 nm. This is in the ultra-violet and so there would be no visible sign of any light being absorbed - buta-1,3-diene is colourless. You read the symbol on the graph as "lambda-max".
What do peaks in UV-Vis mean?
Many organic compounds give more than one maximum peak when its UV-Vis spectra is analyzed. Each peak correspond to a electron transition from a ground state to an excited state, and more than one different transitions (with different energy, and therefore, different wavelenght) are allowed.
Why are the peaks in UV spectrum broad?
In UV-Visible spectra Bonds will be in constant vibration, this variation will absorb nearby energies i.e, ΔE , for this reason UV peaks are broader. Spectrum is broaden by spontaneous emission.
How do you read a color spectrum?
4:5411:11Visible Light Spectrum Explained - Wavelength Range / Color Chart ...YouTubeStart of suggested clipEnd of suggested clipGreen light ranges from 500 to about 570.. And yellow is very small it's 570 to 590. The range isMoreGreen light ranges from 500 to about 570.. And yellow is very small it's 570 to 590. The range is limited orange goes up from like 590 to 620. So red is from 620 to 700.
How do you read a spectral line?
11:0312:20Properties of Light: Spectral Lines 1 - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf you heat up helium. And look at the light that's given off it'll give a different set of spectralMoreIf you heat up helium. And look at the light that's given off it'll give a different set of spectral lines and sodium.
What is peak absorbance?
In the field of spectroscopy, the frequency or wavelength of a given sample which exhibits the maximum or the highest spectral value of absorption.
How are wavelength and absorbance related?
One important consideration is the wavelength of radiation to use for the measurement. Remember that the higher the molar absorptivity, the higher the absorbance. What this also means is that the higher the molar absorptivity, the lower the concentration of species that still gives a measurable absorbance value.
What is lambda max in UV spectroscopy?
Lambda max (λmax): The wavelength at which a substance has its strongest photon absorption (highest point along the spectrum's y-axis). This ultraviolet-visible spectrum for lycopene has λmax = 471 nm.
What do absorption lines tell us?
The absorption lines produced by these outermost layers of the star tell us a lot about the chemical compositition, temperature, and other features of the star.
How do you interpret the differences in the width of absorption lines?
For unresolved lines, one can measure the equivalent width, EW as follows. The equivalent width is equal to the area between the line and the continuum. For an absorption line, the equivalent width is equal to the width of a pure black line (F o = 0), with the same total flux as that absorbed by the line.
What is absorption spectrum simple definition?
Definition of absorption spectrum : an electromagnetic spectrum in which a decrease in intensity of radiation at specific wavelengths or ranges of wavelengths characteristic of an absorbing substance is manifested especially as a pattern of dark lines or bands.
What is the UV absorbance of 4-methyl-3-penten-2-one?
The conjugated pi system in 4-methyl-3-penten-2-one gives rise to a strong UV absorbance at 236 nm due to a π - π * transition. However, this molecule also absorbs at 314 nm. This second absorbance is due to the transition of a non-bonding (lone pair) electron on the oxygen up to a π * antibonding MO:
What is the simplest conjugated system?
Let’s revisit the MO picture for 1,3-butadiene, the simplest conjugated system (see section 2.1B ). Recall that we can draw a diagram showing the four pi MO’s that result from combining the four 2p z atomic orbitals. The lower two orbitals are bonding, while the upper two are antibonding.
What happens to the energy gap of conjugated pi systems?
As conjugated pi systems become larger, the energy gap for a π - π * transition becomes increasingly narrow, and the wavelength of light absorbed correspondingly becomes longer. The absorbance due to the π - π * transition in 1,3,5-hexatriene, for example, occurs at 258 nm, corresponding to a Δ E of 111 kcal/mol.
What is the absorbance of 260 nm?
You can see that the absorbance value at 260 nm (A 260) is about 1.0 in this spectrum.
When a double-bonded molecule such as ethene absorbs light, it undergoes?
When a double-bonded molecule such as ethene (common name ethylene) absorbs light, it undergoes a π - π* transition. Because π - π * energy gaps are narrower than σ - σ* gaps, ethene absorbs light at 165 nm - a longer wavelength than molecular hydrogen.
Is a n- transition weaker than a p orbital?
In general, n - π * transitions are weaker ( less light absorbed) than those due to π - π * transitions.
How many orbitals does buta-1,3-diene have?
In buta-1,3-diene, there are two pi bonding orbitals and two pi anti-bonding orbitals. This is all discussed in detail on the introductory page that you should have read. The highest occupied molecular orbital is often referred to as the HOMO - in these cases, it is a pi bonding orbital.
What happens to the energy of each wavelength of light?
If that particular amount of energy is just right for making one of these energy jumps, then that wavelength will be absorbed - its energy will have been used in promoting an electron.
Why does absorption take place over a range of wavelengths?
This problem arises because rotations and vibrations in the molecule are continually changing the energies of the orbitals - and that, of course, means that the gaps between them are continually changing as well. The result is that absorption takes place over a range of wavelengths rather than at one fixed one.
What happens when light passes through a compound?
When light passes through the compound, energy from the light is used to promote an electron from a bonding or non-bonding orbital into one of the empty anti-bonding orbitals.
Why does the graph look like it does with a broad absorption peak rather than a single line at 217?
If you are really wide-awake you might wonder why the graph looks like it does with a broad absorption peak rather than a single line at 217 nm. A jump from a pi bonding orbital to a pi anti-bonding orbital ought to have a fixed energy and therefore absorb a fixed wavelength. The compound is in fact absorbing over a whole range of wavelengths suggesting a whole range of energy jumps.
What wavelength do jumps absorb?
The jumps shown with grey dotted arrows absorb UV light of wavelength less that 200 nm.
What happens if you have a bigger energy jump?
If you have a bigger energy jump, you will absorb light with a higher frequency - which is the same as saying that you will absorb light with a lower wavelength. Important summary. The larger the energy jump, the lower the wavelength of the light absorbed.
What is the absorption of benzene?
Benzene exhibits very strong light absorption near 180 nm (ε > 65,000) , weaker absorption at 200 nm (ε = 8,000) and a group of much weaker bands at 254 nm (ε = 240). Only the last group of absorptions are completely displayed because of the 200 nm cut-off characteristic of most spectrophotometers. The added conjugation in naphthalene, anthracene and tetracene causes bathochromic shifts of these absorption bands, as displayed in the chart on the left below. All the absorptions do not shift by the same amount, so for anthracene (green shaded box) and tetracene (blue shaded box) the weak absorption is obscured by stronger bands that have experienced a greater red shift. As might be expected from their spectra, naphthalene and anthracene are colorless, but tetracene is orange.
Why is it important to correct the absorbance value?
Because the absorbance of a sample will be proportional to the number of absorbing molecules in the spectrometer light beam (e.g. their molar concentration in the sample tube), it is necessary to correct the absorbance value for this and other operational factors if the spectra of different compounds are to be compared in a meaningful way. The corrected absorption value is called "molar absorptivity", and is particularly useful when comparing the spectra of different compounds and determining the relative strength of light absorbing functions (chromophores). Molar absorptivity (ε) is defined as:
What wavelength is chromophores detected?
The presence of chromophores in a molecule is best documented by UV-Visible spectroscopy, but the failure of most instruments to provide absorption data for wavelengths below 200 nm makes the detection of isolated chromophores problematic.
What were the colors of early humans?
Early humans valued colored pigments, and used them for decorative purposes. Many of these were inorganic minerals, but several important organic dyes were also known. These included the crimson pigment, kermesic acid, the blue dye, indigo, and the yellow saffron pigment, crocetin.
What is the longest visible wavelength?
The longest visible wavelength is red and the shortest is violet. Other common colors of the spectrum, in order of decreasing wavelength, may be remembered by the mnemonic: ROY G BIV. The wavelengths of what we perceive as particular colors in the visible portion of the spectrum are displayed and listed below.
What is the wavelength of a wave?
Visible wavelengths cover a range from approximately 400 to 800 nm. The longest visible wavelength is red and the shortest is violet.
What is the difference between quinone and chlorophyll?
1. Background. An obvious difference between certain compounds is their color. Thus, quinone is yellow; chlorophyll is green; the 2,4-dinitrophenylhydrazone derivatives of aldehydes and ketones range in color from bright yellow to deep red, depending on double bond conjugation; and aspirin is colorless.
