Find out the highest absorbance value and divide each absorbance by that number. For instance, if the highest peak absorbance was 1.05 in the whole spectrum, divide each absorbance value by 1.05 in excel and re-plot the spectrum. Normalization is helpful when the spectrum is changing in its shape or the peaks are shifting on the wavelength scale.
Full Answer
How to normalize the area of a spectrophotometer?
Normalization of total intensity (area normalization) For some purposes, such as decomposing spectra, having the area-normalized spectra of the individual components is convenient. 1. Go to Tools->'Normalize spectra (area)'. 2. A spectrum selection window is opened. Select the spectra to normalize and press OK.
How do I normalize selected spectra and wavelengths?
Press the 'Normalize selected spectra' button in the toolbar. If no spectra and wavelengths are selected using the data cursor tool a selection dialog box will be opened. Select the desired spectra in the dialog box and press OK.
How many peaks does a UV-Vis spectrum have?
You’ll notice that this UV spectrum is much simpler than the IR spectra we saw earlier: this one has only one peak, although many molecules have more than one. Notice also that the convention in UV-vis spectroscopy is to show the baseline at the bottom of the graph with the peaks pointing up.
What is the convention in UV-Vis spectroscopy?
Notice also that the convention in UV-vis spectroscopy is to show the baseline at the bottom of the graph with the peaks pointing up. Wavelength values on the x-axis are generally measured in nanometers (nm) rather than in cm -1 as is the convention in IR spectroscopy.
How do you normalize absorption spectra?
Find out the highest absorbance value and divide each absorbance by that number. For instance, if the highest peak absorbance was 1.05 in the whole spectrum, divide each absorbance value by 1.05 in excel and re-plot the spectrum.
How do you normalize peak intensity?
Peak normalization - Choose the peak with maximum intensity and divide all other peak intensities with the maximum peak intensity....All Answers (8)take maximum intensity value what you get in recording.now devide each intensity value with that maximum value.then all intensity values are in between 0 to 1.
How do you normalize fluorescence intensity?
One way to normalize fluorescence intensity data from time-lapse imaging is by dividing the intensity at every time-point (I) by the fluorescence intensity of the first time point (I0). One application of this normalization method is for analyzing and comparing photostability.
Why do we need to Normalise Spectra?
Normalization at one wavelength For plotting purposes spectra can be rescaled so that the absorption/intensity value at a certain wavelength is 1. In this example, three emission spectra are normalized at different wavelengths.
How do you normalize a curve?
Normalize data to the standard normal distribution. Divide the column or curve by the dataset maximum value. Divide the column or curve by the dataset minimum value. Divide the column or curve by the dataset mean value.
How do you normalize a signal?
Normalizing the amplitude of a signal is to change the amplitude to meet a particular criterion. One type of normalization is to change the amplitude such that the signal's peak magnitude equals a specified level. Peak amplitude is a measurement based the instantaneous level of a signal.
How do you normalize to baseline?
To normalize, click the Analyze button in the Analysis section of the toolbar. Then select Normalize from the "Transform, Normalize..." section of the analyses at the top of the list. Click OK which will bring up the Parameters: Normalize dialog. To normalize between 0 and 100%, you must define these baselines.
How do you normalize data?
Here are the steps to use the normalization formula on a data set:Calculate the range of the data set. ... Subtract the minimum x value from the value of this data point. ... Insert these values into the formula and divide. ... Repeat with additional data points.
How do you normalize an experimental data?
- Divide all individual values for each experiment by its mean value to obtain a normalized value (experiment normalized). Your values will now be centered around one.
What is a normalized spectrum?
The spectrum of a signal is the square of the Fourier transform of the signal. The spectral estimate using the commands spa , spafdr , and etfe is normalized by the sample time T: Φ y ( ω ) = T ∑ k = − M M R y ( k T ) e − i w T W M ( k )
What is spectrum normalization?
Spectral Normalization is a weight normalization that stabilizes the training of the discriminator. It controls the Lipschitz constant of the discriminator to mitigate the exploding gradient problem and the mode collapse problem.
What is Normalisation?
Normalization is the process of organizing data in a database. This includes creating tables and establishing relationships between those tables according to rules designed both to protect the data and to make the database more flexible by eliminating redundancy and inconsistent dependency.
What is peak normalization?
Peak normalization detects an audio file's maximum (peak) level and then raises (or lowers) it to a target peak level. That target peak level is often 0dBFS (maximum available value) but could be a different level, like 6dB below the maximum value.
How does loudness normalization work?
Loudness normalization adjusts the recording based on perceived loudness. Normalization differs from dynamic range compression, which applies varying levels of gain over a recording to fit the level within a minimum and maximum range. Normalization adjusts the gain by a constant value across the entire recording.
How do you normalize value?
To normalize the values in a dataset to be between 0 and 100, you can use the following formula:zi = (xi – min(x)) / (max(x) – min(x)) * 100.zi = (xi – min(x)) / (max(x) – min(x)) * Q.Min-Max Normalization.Mean Normalization.
What is normalization in signal processing?
In digital signal processing (DSP), normalized frequency (f') is a quantity having dimension of frequency expressed in units of "cycles per sample". It equals f'=f/fs, where f is an ordinary frequency quantity (in "cycles per second") and fs is the sampling rate (in "samples per second").
How to normalize spectra in a spectrometer?
1. Go to Tools->'Normalize spectra (area)'. 2. A spectrum selection window is opened. Select the spectra to normalize and press OK. The selected spectra are now normalized to a total area of 1. You can use the integration tool to verify this.
What is a|e in spectroscopy?
For plottting and analysis purposes a|e provides a one-click shortcut to normalizing UV-Vis absorption and emission spectra, either normalization at a single wavelength or normalization of the area below the spectrum.
Does normalization take place at the wavelength?
Since no spectra were selected using the data cursor tool, normalization will in this case be done at the wavelength corresponding to the peak value of the spectra.
Can emission spectra be rescaled?
For plotting purposes spectra can be rescaled so that the absorption/intensity value at a certain wavelength is 1. In this example, three emission spectra are normalized at different wavelengths.
How is absorbance measured?
Absorbance measurements are frequently used to quantify an unknown sample’s concentration by exploiting the Beer-Lambert Law that describes how light is attenuated based on the materials it passes through. The transmittance, and therefore the absorbance, are directly proportional to a sample’s concentration, c, molar absorptivity, ε , and cuvette pathlength, l.
What type of light source is used in a spectrophotometer?
JASCO spectrophotometers use deuterium and halogen light sources. The deuterium lamp is used for the UV region from 190 to 350 nm while the halogen lamp covers a much broader spectral range from 330 and 3200 nm. Both the deuterium and halogen lamps used are continuous sources, although the D2 is also a line source. In continuous sources, the arc created excites the molecules enclosed in the vacuum to a higher energy state. The relaxation of the electrons back to the ground state emits photons and as the electrons return to the ground state, the excitation process restarts, providing a continuous source of light. Continuous sources therefore provide a uniform amount of light through the monochromator to the sample. While this constant output of light can potentially lead to photobleaching of light sensitive samples, shutters can be implemented so the sample is only irradiated with light during the measurement itself.
How does a monochromator work?
The monochromator itself houses the mirrors, slits, and grating. panchromatic light from a light source is introduced into the monochromator through the entrance slit and collimated onto a diffraction grating which is rotated to select discrete wavelengths. The light is then refocused by another mirror onto the exit slit so that can be adjusted to control the spectral bandwidth (SBW). The light is then refocused by another series of mirrors and directed to the sample where it is either transmitted, absorbed, or reflected.
What are the two types of optical arrangements?
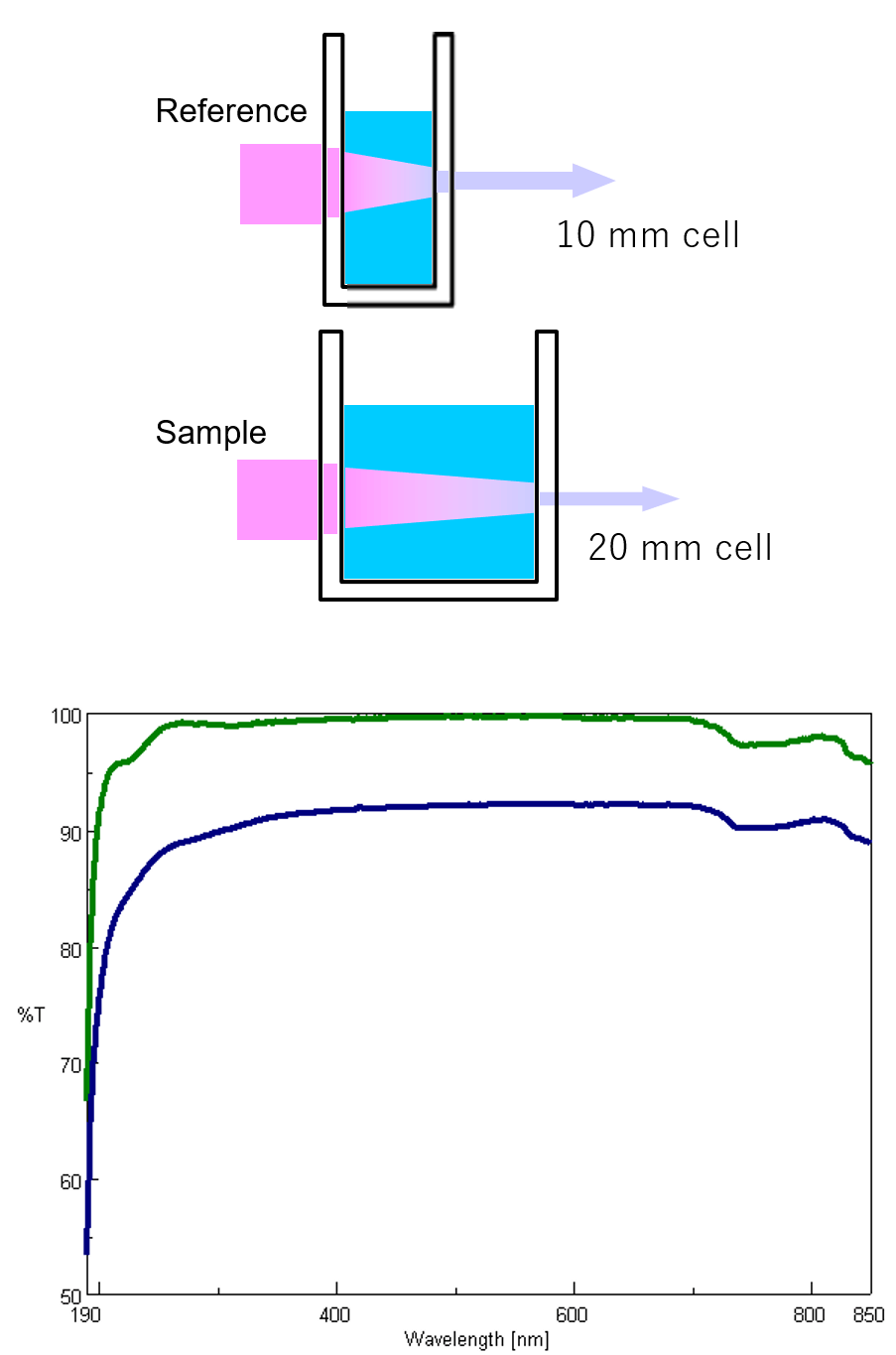
There are two types of optical arrangements: single and double beam. In the single-beam configuration, the monochromator, sample, and detector are arranged in series and the obtained monochromatic light with intensity I0 irradiates a sample and the transmitted light with intensity I is detected. Here, I/I0 is the transmittance. Although the optical system is simple, it is easily affected by fluctuations in the light source, and a blank measurement is required for each measurement. In a double beam configuration, monochromatic light is divided into two beams by a fixed or dynamic beam splitter, and the individual beams pass through a sample and a reference and detected, shown in figure 6. The light source intensity changes with time so the reference beam monitors the lamp energy and accounts for energy differences from voltage fluctuations, lamp drift, and stray light. By splitting the optical path, the incident and transmitted light can be measured simultaneously, compensating for the effects of light source fluctuations. Therefore, the measured absorbance is the ratio of the sample beam to the reference beam.
How do PMT detectors work?
In a photomultiplier tube (PMT) detector, photons are incident on the photocathode surface which produces electrons. The initial electrons travel through the tube where they hit a series of plates or dynodes that amplify the number of electrons for every dynode that’s hit via secondary emission. The multiplied secondary electrons are collected at the anode, sent to an external circuit, and converted to the output signal. PMTs have a wide spectral response, high signal to noise output, and high stability.
What is the color of the double beam instrument?
Figure 6. Double beam instrument schematic. The beam splitter is highlighted in yellow, the reference beam in blue, and the sample beam is highlighted in green.
How to differentiate peaks in a spectrum?
To differentiate peaks in a spectrum, the bandwidths and therefore the slit widths, need to be adjusted. The instrument’s ability to separate light into defined wavelength regions is known as spectral resolution. The narrower the bandwidth and therefore the slits, the better the resolution. However, since less light will pass through narrower slits, the spectrum will have more noise. Likewise, wider slits and bandwidths increase the light throughput and therefore the signal, but produce poorer peak resolution. The goal is to find a balance between the required resolution and the desired signal to noise. Figure 9 illustrates the measured absorption spectra using bandwidths of 1 and 5 nm. As the bandwidth increases, the peaks collapse and broaden.
What is the ratio of A-Amin to Amax-Amin?
The ratio (A-Amin)/ (Amax-Amin), usually (A at 280 nm )/ (A at 254 nm) is a unique value for each protein and can be used as a criterion for protein purity.
Why join Researchgate?
Join ResearchGate to find the people and research you need to help your work.
When is normalization helpful?
Normalization is helpful when the spectrum is changing in its shape or the peaks are shifting on the wavelength scale. It may not be wise to normalize the spectrum if the peak intensity was supposed to change with pH. Cite. 8 Recommendations. 22nd Mar, 2014.
Can you normalize spectra if you have independent solubility data vs. pH?
If you have independent solubility data vs. pH, you can normalize the spectra to identical concentration. If not, it is not easy.
What is the UV absorbance of 4-methyl-3-penten-2-one?
The conjugated pi system in 4-methyl-3-penten-2-one gives rise to a strong UV absorbance at 236 nm due to a π – π* transition. However, this molecule also absorbs at 314 nm. This second absorbance is due to the transition of a non-bonding (lone pair) electron on the oxygen up to a π* antibonding MO:
What is the simplest conjugated system?
Let’s revisit the MO picture for 1,3-butadiene, the simplest conjugated system (see section 2.1B ). Recall that we can draw a diagram showing the four pi MO’s that result from combining the four 2p z atomic orbitals. The lower two orbitals are bonding, while the upper two are antibonding.
What happens to the energy gap of conjugated pi systems?
As conjugated pi systems become larger, the energy gap for a π – π* transition becomes increasingly narrow, and the wavelength of light absorbed correspondingly becomes longer. The absorbance due to the π – π* transition in 1,3,5-hexatriene, for example, occurs at 258 nm, corresponding to a Δ E of 111 kcal/mol.
Why are carrots orange?
Beta-carotene, with its system of 11 conjugated double bonds, absorbs light with wavelengths in the blue region of the visible spectrum while allowing other visible wavelengths – mainly those in the red-yellow region – to be transmitted. This is why carrots are orange.
What is the absorbance of 260 nm?
You can see that the absorbance value at 260 nm (A 260) is about 1.0 in this spectrum.
What is the range of UV spectroscopy?
The electronic transitions of both molecular hydrogen and ethene are too energetic to be accurately recorded by standard UV spectrophotometers, which generally have a range of 220 – 700 nm. Where UV-vis spectroscopy becomes useful to most organic and biological chemists is in the study of molecules with conjugated pi systems.
When a double-bonded molecule such as ethene absorbs light, it undergoes?
When a double-bonded molecule such as ethene (common name ethylene) absorbs light, it undergoes a π – π* transition. Because π- π* energy gaps are narrower than σ – σ * gaps, ethene absorbs light at 165 nm – a longer wavelength than molecular hydrogen.
How to normalize a peak?
1. Peak normalization - Choose the peak with maximum intensity and divide all other peak intensities with the maximum peak intensity. 2. Area normalization - Choose the peak with maximum peak area and divide all other peak areas with the maximum peak area. The procedure is similar to peak normalization but instead of dividing peak intensity ...
How to find the maximum value of a data file?
1. Take the two data files in the software of your choice. 2. Find the maxima of the y-column of both the data files using "max" function (in excel it is the max function or equivalent function in other software) 3. Divide the entire y-column of both the data files with their corresponding maximum value. 4.
What does the max function return?
Now you have the data which has been normalized. The max function will return "1.0" as the maximum value for these data sets.
Which is the most accurate method of estimating area normalization?
Area normalization by integration is the most accurate but cumbersome relatively.
Does normalizing to the peak yield a peak of 1?
Normalizing to the peak value does indeed yield a peak of 1 for both spectra, but the background intensity is not the same (See middle plot). To account for this, you need to first subtract the background counts (in my case a constant background is sufficient) before doing the normalization. After doing this, now both spectra are normalized ...
What is the absorption of benzene?
Benzene exhibits very strong light absorption near 180 nm (ε > 65,000) , weaker absorption at 200 nm (ε = 8,000) and a group of much weaker bands at 254 nm (ε = 240). Only the last group of absorptions are completely displayed because of the 200 nm cut-off characteristic of most spectrophotometers. The added conjugation in naphthalene, anthracene and tetracene causes bathochromic shifts of these absorption bands, as displayed in the chart on the left below. All the absorptions do not shift by the same amount, so for anthracene (green shaded box) and tetracene (blue shaded box) the weak absorption is obscured by stronger bands that have experienced a greater red shift. As might be expected from their spectra, naphthalene and anthracene are colorless, but tetracene is orange.
Why is it important to correct the absorbance value?
Because the absorbance of a sample will be proportional to the number of absorbing molecules in the spectrometer light beam (e.g. their molar concentration in the sample tube), it is necessary to correct the absorbance value for this and other operational factors if the spectra of different compounds are to be compared in a meaningful way. The corrected absorption value is called "molar absorptivity", and is particularly useful when comparing the spectra of different compounds and determining the relative strength of light absorbing functions (chromophores). Molar absorptivity (ε) is defined as:
What wavelength is chromophores detected?
The presence of chromophores in a molecule is best documented by UV-Visible spectroscopy, but the failure of most instruments to provide absorption data for wavelengths below 200 nm makes the detection of isolated chromophores problematic.
What were the colors of early humans?
Early humans valued colored pigments, and used them for decorative purposes. Many of these were inorganic minerals, but several important organic dyes were also known. These included the crimson pigment, kermesic acid, the blue dye, indigo, and the yellow saffron pigment, crocetin.
What is the longest visible wavelength?
The longest visible wavelength is red and the shortest is violet. Other common colors of the spectrum, in order of decreasing wavelength, may be remembered by the mnemonic: ROY G BIV. The wavelengths of what we perceive as particular colors in the visible portion of the spectrum are displayed and listed below.
What is the wavelength of a wave?
Visible wavelengths cover a range from approximately 400 to 800 nm. The longest visible wavelength is red and the shortest is violet.
What is the difference between quinone and chlorophyll?
1. Background. An obvious difference between certain compounds is their color. Thus, quinone is yellow; chlorophyll is green; the 2,4-dinitrophenylhydrazone derivatives of aldehydes and ketones range in color from bright yellow to deep red, depending on double bond conjugation; and aspirin is colorless.
