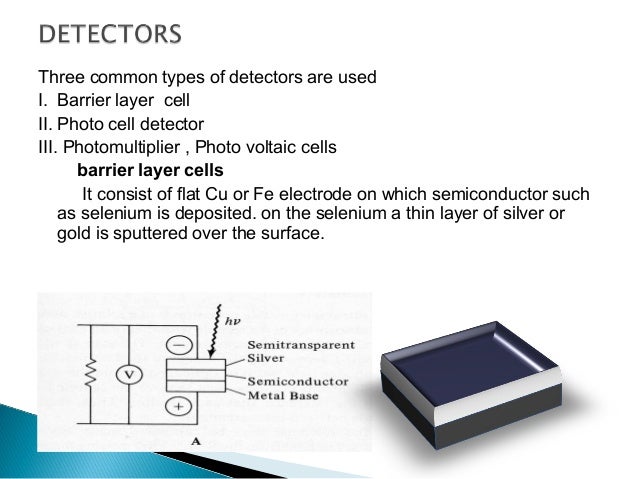
How to use the UV-Vis spectrophotometer correctly?
- It is advisable to put the solution into the cuvette at a height of about 2/3; try to avoid the generation of...
- When the cuvette is used, it should be cleaned in time and cleaned by appropriate methods. If it is improperly...
- During the test, if a light source is not used, you can choose to turn it off to...
What is UV Vis spectra?
UV-Vis Spectroscopy (or Spectrophotometry) is a quantitative technique used to measure how much a chemical substance absorbs light. This is done by measuring the intensity of light that passes through a sample with respect to the intensity of light through a reference sample or blank.
What is the UV Vis spectrum?
UV-vis spectroscopy is a cost-effective, simple, versatile, non-destructive, analytical technique suitable for a large spectrum of organic compounds and some inorganic species. As a function of wavelength, UV-vis spectrophotometers measure the absorption or transmission of light that passes through a medium. In order to classify and measure the ...
What are some uses of UV/Vis spectroscopy?
13.21.1.1: Some Uses of UV/Vis Spectroscopy
- Electronic transitions. Let's take as our first example the simple case of molecular hydrogen, H 2. ...
- Looking at UV-vis spectra. We have been talking in general terms about how molecules absorb UV and visible light - now let's look at some actual examples of data from ...
- Applications of UV spectroscopy in organic and biological chemistry
How does UV Vis spectroscopy work?
How does UV-Vis absorption spectroscopy work? A UV-Vis spectrophotometer measures the intensity of light transmitted through a sample compared to a reference measurement of the incident light source. The transmitted light is acquired by a CCD optical detector with a wavelength accuracy of within 0.5nm.

What is UV spectrophotometer?
UV-visible spectrophotometers are precision instruments. The performance of electronic and optical components may change during operation due to working environment and operation methods, which may further affect equipment performance and even cause equipment failure or accident.
How high should a cuvette be?
1. It is advisable to put the solution into the cuvette at a height of about 2/3; try to avoid the generation of bubbles; if the solution remains on the outer wall of the cuvette, wipe it off in time (first use filter paper to dry, then wipe with mirror paper) Glossy); for volatile samples, it is recommended to use a cuvette cover;
What happens if a cuvette is not cleaned?
If it is improperly cleaned or not cleaned, it will affect the accuracy and stability of the measurement results. 3.
What to do if a light source is not used?
During the test, if a light source is not used, you can choose to turn it off to extend the service life of the lamp. When the instrument is not in use, please turn off the power and unplug the power plug to prevent damage caused by thunderstorms. 4.
What is Principle of UV-Visible Spectrophotometer?
When a beam of monochromatic light is passing through a homogeneous absorbing medium the rate of decrease in intensity of light is directly proportional to the concentration and path length of the absorbing medium.
What wavelength is used for spectrophotometry?
The wavelength range that is typically used for spectrophotometry, despite being referred to as UV-Vis, is 190 nm in the near infrared to 1,100 nm in the ultraviolet. By measuring the number of photons that reach a spectrophotometer and carrying out absorption/transmission measurements, we can just about determine the concentration (factor) ...
How many wavelengths does a blank absorb?
The blank absorbs at only one wavelength, or at a particular range of wavelengths, but not more, and that absorbance is subtracted from the sample’s absorbance. Once the blank is discarded and the cuvette has been rinsed twice with sample, fill the cuvette about 75% full with sample.
What is UV/VIS spectroscopy?
Ultraviolet and visible light range (UV/VIS) is widely applied in research, production and quality control for the classification and study of substances. UV/VIS spectroscopy is based on the absorption of light by a sample. Depending on the amount of light and its wavelength absorbed by the sample, valuable information can be obtained, such as the purity of the sample. Moreover, the amount of absorbed light is related to the amount of sample, and thus, quantitative analysis is possible by optical spectroscopy. This article more specifically explores techniques when using a spectrophotometer to determine concentration of an analyte. A UV/VIS spectrophotometer measures the intensity of light passing through a sample solution in a cuvette, and compares it to the intensity of the light before it passes through the sample. The main components of a UV/VIS spectrophotometer are a light source, a sample holder, a dispersive device to separate the different wavelengths of the light and a suitable detector. This instrument measures Transmittance which is the ratio of the transmitted intensity I to the original intensity of light. An important derived (calculated) variable also reported by the instrument is the Absorbance which is defined as A = −log (Transmittance).
When using a spectrophotometer to determine concentration of a sample solution of unknown concentration by UV/VIS?
When using a spectrophotometer to determine concentration of a sample solution of unknown concentration by UV/VIS spectroscopy, a calibration line must first be created . This is done by measuring the light absorption of several standard solutions of different , known concentrations at a predefined, fixed wavelength. The below calibration line is obtained:
How do scanning spectrophotometers measure transmittance?
The grating is rotated in order to individually select each wavelength that is then sent through a cuvette. The transmittance at this specific wavelength is recorded. The whole spectrum is obtained by continuously changing the wavelength of light (i.e. scanning) incoming onto the sample solution by rotating the grating. Alternately, in Array Spectrophotometers, the sample is illuminated by a light beam consisting of all spectral components of the UV/ VIS range. The sample in the cuvette absorbs all wavelengths simultaneously and the transmitted light is diffracted and then detected by a CCD sensor. Measuring the whole UV/VIS spectrum is generally faster than using a conventional scanning spectrophotometer since the spectrum is recorded simultaneously at all wavelengths. Moreover, an array detector has an integrating function which accumulates individual measurements to enhance the signal, leading to a strongly increased signal to noise ratio, and thus to an improved signal quality of the measured spectrum. Array Spectrophotometers present an innovative approach to speed up full spectrum scan based on reverse optics technology. The robust design without any moving optical parts ensures very good optical performance.
What is the wavelength of a Xenon flash lamp?
The light source consists of a Xenon flash lamp for the ultraviolet (UV) as well as for the visible (VIS) and near-infrared wavelength regions covering a spectral range from 190 up to 1100 nm. The lamp flashes are focused on a glass fiber which drives the beam of light onto a cuvette containing the sample solution.
Is UV absorbance a function of wavelength?
Absorbance as a function of wavelength. In general, a UV/VIS spectrum is graphically represented as absorbance as a function of wavelength. The advantage of this representation is obvious; the height of the absorption peaks is directly proportional to the concentration of the species. The calculation of concentration is governed by ...
What is the wavelength of a UV spectrophotometer?
Ultraviolet visible (UV-Vis) spectrophotometers use a light source to illuminate a sample with light across the UV to the visible wavelength range (typically 190 to 900 nm). The instruments then measure the light absorbed, transmitted, or reflected by the sample at each wavelength. Some spectrophotometers have an extended wavelength range, into the near-infrared (NIR) (800 to 3200 nm).
What light source is used for UV spectroscopy?
Unfortunately, such a source does not exist. Two different light sources have historically been used in UV-visible spectrophotometers: – The deuterium arc lamp was used to provide a good intensity continuum in the UV region and useful intensity in the visible region – The tungsten-halogen lamp yielded good intensity over the entire visible range and part of the UV spectrum More recently, a single Xenon flash lamp has been used more widely. The use of a Xenon flash lamp as a single source has significant advantages over the use of the two conventional lamps. Deuterium (D
How does a monochromator work?
To narrow the light down to a selected wavelength band, the light is passed through a monochromator. A monochromator consists of: – An entrance slit, – A dispersion device, to spread the light into different wavelengths (like a rainbow) and allow the selection of a nominated band of wavelengths, and – An exit slit where the light of the nominated wavelengths passes through and onto the sample. An easy way to think about a monochromator is to think of a room, with the sun shining through a window. The sunlight hits a prism that separates the white light into a rainbow. The rainbow falls onto a window on the opposite side of the room. As the prism is turned, light of different colors i.e. different wavelengths, pass out of the room through the window. Ideally, the output from a monochromator is light of a single wavelength. In practice, however, the output is always a band of wavelengths. Most spectrophotometers on the market today contain holographic gratings as the dispersion device. These optical components are made from glass, onto which extremely narrow grooves are precisely etched onto the surface. The dimensions of the grooves are of the same order as the wavelength of light to be dispersed. Finally, an aluminum coating is applied to create a reflective surface. Interference and diffraction of the light falling on the grating is reflected at different angles, depending on the wavelength. Holographic gratings yield a linear angular dispersion with wavelength and are temperature insensitive. However, they reflect light in different orders, which overlap (see Figure 12). As a result, filters must be used to ensure that only the light from the desired reflection order reaches the detector. A concave grating disperses and focuses light simultaneously.
What is a single monochromator spectrophotometer?
A single monochromator spectrophotometer is used for general-purpose spectroscopy and can be integrated into a compact optical system. Figure 13 shows a schematic diagram of a single monochromator optical system. A single monochromator spectrophotometer cannot select the wavelengths of light as narrowly as a double monochromator system, but this ability may not be required for many applications, for example when measuring samples that have broad absorption peaks.
How to determine the wavelength of electromagnetic radiation?
Because radiation acts as a wave, it can be classified in terms of either waveleng th or frequency, which are related by the following equation: ν = c/λ where ν is frequency (in seconds), c is the speed of light (3 × 108ms-1), and λ is wavelength (in meters). In UV-Vis spectroscopy, wavelength is usually expressed in nanometers (1 nm = 10-9m). It follows from the equations that radiation with shorter wavelength has higher energy, and, for UV-Vis spectroscopy, the low (short)
What is a tungsten halogen lamp?
Tungsten-halogen lamp The tungsten-halogen lamp uses a filament. When a current is passed through the filament, it becomes heated and emits light (see Figure 10). The lamp yields good intensity over part of the UV spectrum and over the entire visible and NIR range (350 nm- 3000 nm). This type of lamp has very low noise and low drift and typically has a functional life of 10,000 h.
When light passes through or is reflected from a sample, the amount of light absorbed is the difference between the?
When light passes through or is reflected from a sample, the amount of light absorbed is the difference between the incident radiation (I
