What is UV Vis spectroscopy?
What is UV VIS Spectroscopy? Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region.
Can UV spectroscopy be used to identify compounds?
UV–vis spectroscopy is relatively weak at identifying compounds; it is much more useful in quantitative assessments. In SMP characterization, UV–vis spectroscopy is an especially effective method for measuring basic optical characteristics of a sample and the concentrations of its known extractables or degradation products.
What is the light source of UV-Vis spectrophotometer?
In UV-vis spectroscopy, the light source is usually a single xenon lamp that is capable of both UV and visible light wavelengths. However, some UV-vis spectrophotometers implement a dual light source solution — a tungsten or halogen lamp, which emits the visible light, and a deuterium lamp, which emits the UV light.
What is UV 3 spectroscopy used for?
3.2.1Ultraviolet–visible spectroscopy Ultraviolet–visible (UV–vis) spectroscopy is a technique most commonly applied to quantitative measurement of solutions, specifically for determining concentrations of known solutes. However, its application to solid-state samples can also provide valuable insights.
Is UV spectroscopy destructive or non-destructive?
For this purpose, the feasibility of UV–Vis Diffuse Reflectance Spectroscopy (UV–Vis DRS) is described as a rapid, inexpensive and non-destructive method of analysis of PM filters.
Is spectrophotometry destructive?
Spectrophotometry provides a non-destructive method of analyzing water for quality, clarity and purity.
What are disadvantages of UV-Vis spectrometry?
The main disadvantage of using a UV-VIS spectrometer is the time it takes to prepare to use one. With UV-VIS spectrometers, setup is key. You must clear the area of any outside light, electronic noise, or other outside contaminants that could interfere with the spectrometer's reading.
Does UV-Vis destroy the sample?
UV-vis spectroscopy is non-invasive and non-destructive, which makes it ideal when analyzing precious or scarce samples. After the procedure, most solutions and compounds can be used as intended.
Which is destructive spectroscopy?
5.01.2.3 Electrochemical Impedance Spectroscopy. While potentiodynamic polarization is typically considered a destructive technique, that is, it alters the surface of the corrosion sample, electrochemical impedance spectroscopy (EIS) is a powerful nondestructive technique for obtaining a wealth of data including Rp.
Which detecting technique is destructive?
This includes different types of destructive testing methods such as tension tests, bend tests, Charpy impact tests, Pellini drop weight testing, peel tests, crush testing, pressure and fracture testing.
What is the main strength and main weakness of ultraviolet Spectrophotometry?
The main strength of UV spectrophotometry is that the spectra it produces are very simple, which makes determining a material's probable identity easier. The main drawback of UV spectrophotometry is that UV spectra of different substances can be similar, so the technique may not provide a definitive result.
What are the limitations of Spectrophotometry?
Spectrophotometry is a conventional and inexpensive technique. However, it also has several limitations, including low sensitivity and selectivity. Spectrophotometric determination of iodate in seawater involved the reaction of with excess I− under acid conditions to form I2.
What is a disadvantage of spectroscopy?
Cannot be used for metals or alloys. The Raman effect is very weak, which leads to low sensitivity, making it difficult to measure low concentrations of a substance.
Why does UV light damage DNA?
UVA (and also UVB) radiation cause indirect damage to DNA via absorption of photons by non-DNA chromophores. This generates reactive oxygen species like singlet oxygen or hydrogen peroxide that oxidize the DNA bases causing mutations.
Why glass is not suitable for UV spectroscopy?
Glass is useful when working with colored substances. The reason why glass cuvettes are not suitable for UV spectroscopy is because it absorbs strongly in the UV region, and is therefore not recommended when working for wavelengths below 340 nm. Glass is useful when working with colored substances.
How does UV light denature proteins?
By illuminating two UV-absorbing amino acids, i.e., tryptophan (Trp), near this bond with UV light, we found that this disulfide bond breaks apart in approximately 2 microseconds, and the protein immediately unravels.
What are the limitations in using spectrophotometer?
Spectrophotometry is a conventional and inexpensive technique. However, it also has several limitations, including low sensitivity and selectivity. Spectrophotometric determination of iodate in seawater involved the reaction of with excess I− under acid conditions to form I2.
What are the common mistakes or errors committed when using the spectrophotometer?
In practice there are other sources of error, such as environmental effects on photometer and sample, temperature, line voltage fluctuations, vibrations, contamination, or heating of the sample by the photometer. All these factors may impair the measured result, and ways and means are known to test and eliminate them.
How sensitive is a spectrophotometer?
At a 460 nm wavelength, the sensitivity for the spectrophotometer with an LED is 0.0046 dL/mg, which is 73% higher than that with halogen light that records 0.0012 dL/mg. This enhanced sensitivity is attributed to the higher luminous efficacy of the LED light beam.
What are the disadvantages of single beam spectrophotometer?
DISADVANTAGES: Following are the disadvantages of single beam spectrophotometer: - The primary limitation is, it provides no means to compensate for instrumental variations during an analysis, such as changes in source intensity. - It only measures the absorbance of either sample or reference blank at a time.
Why is UV spectroscopy used in pharmaceutical analysis?
UV spectrophotometers measure the visible regions of ultraviolet light and can provide valuable information, as well as detect any impurities, abou...
What are the applications of spectrophotometry?
In different fields, such as astronomy, molecular biology , chemistry and biochemistry, spectrophotometers are commonly used. Specification applica...
What is the range of UV spectroscopy?
UV-Vis is also considered a general procedure, since in the UV-visible wavelength spectrum, most molecules absorb light. The UV frequency is betwee...
Which lamp is used in UV spectroscopy?
Light with a wavelength range between 190 nm and 800 nm is radiated through the cuvette using a spectrometer and absorption spectrums are recorded....
What is the IR principle?
The principle of IR spectroscopy utilises the idea that molecules appear to absorb unique light frequencies that are typical of the molecules’ corr...
What is UV VIS spectroscopy and how does it work?
UV-Vis is a quick , convenient, and inexpensive way of determining the solution concentration of an analyte. In UV-Vis, a beam travels through a so...
How is absorbance measured?
Absorbance measurements are frequently used to quantify an unknown sample’s concentration by exploiting the Beer-Lambert Law that describes how light is attenuated based on the materials it passes through. The transmittance, and therefore the absorbance, are directly proportional to a sample’s concentration, c, molar absorptivity, ε , and cuvette pathlength, l.
What type of light source is used in a spectrophotometer?
JASCO spectrophotometers use deuterium and halogen light sources. The deuterium lamp is used for the UV region from 190 to 350 nm while the halogen lamp covers a much broader spectral range from 330 and 3200 nm. Both the deuterium and halogen lamps used are continuous sources, although the D2 is also a line source. In continuous sources, the arc created excites the molecules enclosed in the vacuum to a higher energy state. The relaxation of the electrons back to the ground state emits photons and as the electrons return to the ground state, the excitation process restarts, providing a continuous source of light. Continuous sources therefore provide a uniform amount of light through the monochromator to the sample. While this constant output of light can potentially lead to photobleaching of light sensitive samples, shutters can be implemented so the sample is only irradiated with light during the measurement itself.
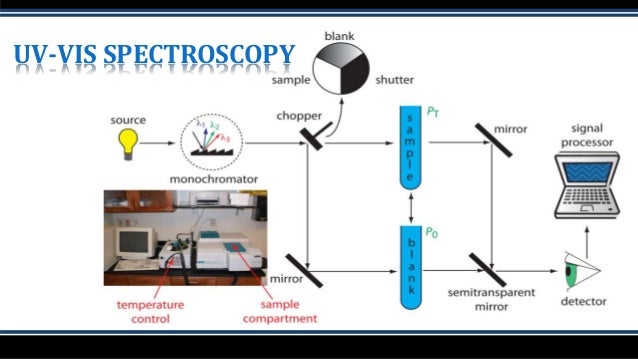
How does a monochromator work?
The monochromator itself houses the mirrors, slits, and grating. panchromatic light from a light source is introduced into the monochromator through the entrance slit and collimated onto a diffraction grating which is rotated to select discrete wavelengths. The light is then refocused by another mirror onto the exit slit so that can be adjusted to control the spectral bandwidth (SBW). The light is then refocused by another series of mirrors and directed to the sample where it is either transmitted, absorbed, or reflected.
What are the two types of optical arrangements?
There are two types of optical arrangements: single and double beam. In the single-beam configuration, the monochromator, sample, and detector are arranged in series and the obtained monochromatic light with intensity I0 irradiates a sample and the transmitted light with intensity I is detected. Here, I/I0 is the transmittance. Although the optical system is simple, it is easily affected by fluctuations in the light source, and a blank measurement is required for each measurement. In a double beam configuration, monochromatic light is divided into two beams by a fixed or dynamic beam splitter, and the individual beams pass through a sample and a reference and detected, shown in figure 6. The light source intensity changes with time so the reference beam monitors the lamp energy and accounts for energy differences from voltage fluctuations, lamp drift, and stray light. By splitting the optical path, the incident and transmitted light can be measured simultaneously, compensating for the effects of light source fluctuations. Therefore, the measured absorbance is the ratio of the sample beam to the reference beam.
How do PMT detectors work?
In a photomultiplier tube (PMT) detector, photons are incident on the photocathode surface which produces electrons. The initial electrons travel through the tube where they hit a series of plates or dynodes that amplify the number of electrons for every dynode that’s hit via secondary emission. The multiplied secondary electrons are collected at the anode, sent to an external circuit, and converted to the output signal. PMTs have a wide spectral response, high signal to noise output, and high stability.
What is the color of the double beam instrument?
Figure 6. Double beam instrument schematic. The beam splitter is highlighted in yellow, the reference beam in blue, and the sample beam is highlighted in green.
How to differentiate peaks in a spectrum?
To differentiate peaks in a spectrum, the bandwidths and therefore the slit widths, need to be adjusted. The instrument’s ability to separate light into defined wavelength regions is known as spectral resolution. The narrower the bandwidth and therefore the slits, the better the resolution. However, since less light will pass through narrower slits, the spectrum will have more noise. Likewise, wider slits and bandwidths increase the light throughput and therefore the signal, but produce poorer peak resolution. The goal is to find a balance between the required resolution and the desired signal to noise. Figure 9 illustrates the measured absorption spectra using bandwidths of 1 and 5 nm. As the bandwidth increases, the peaks collapse and broaden.
How to measure initiation rate?
Various researchers have used UV/visible spectroscopy to acquire initiation rate data. Grubbs and coworkers measured initiation rates for G1and analogues by following the decrease of the absorbance for the precatalyst in the presence of a large excess of EVE (c.750 equivalent)29; phosphine dissociation is rapid (t1/2typically c. 30 s), rendering this reaction too fast to monitor using NMR spectroscopy. Plenio and coworkers, and Percy and coworkers, have used UV/visible spectroscopy to monitor initiation rates for a range of Hoveyda-type precatalysts with different substrates, and in different solvents (vide supra).31,37,52,54–56In some cases, substrate:precatalyst ratios were much greater than 105:1, yet UV/visible spectroscopy can reliably and accurately monitor low concentrations of (highly absorbing) precatalyst species in the presence of a vast excess of substrate.
How to measure analyte interactions with MIPs?
UV/Vis spectroscopyis one of the most simplified and economical methods for examining analyte interactions with MIPs where only the change in absorbance is measured as a function of wavelength. The technique is versatile and gives rapid response regarding quantitative information on template binding. Besides pure sensing application, this method is quitesuitable for screening [36]MIPs and choosing the finest polymer composition. With the help of the UV/Vis spectrum, the thorough mechanism of complexation between templates, monomer, and cross-linker during polymerization can also be better understood. It has been observed that after complexation, an absorbance shift toward shorter wavelengths takes place. The procedure makes it easy to compare the spectrum of free template and functional monomer with that of the complex formed. This strategy is equally suitable for monitoring metal polymer complexation in visible regions [37]. Although UV/Vis spectroscopy is not as selective as the fluorescence method, it is nevertheless quite suitable for designing low-cost MIP sensors with moderate sensitivity.
What is UV visible spectroscopy?
Ultraviolet (UV)-visible spectroscopy is a type of absorption spectroscopy in which UV-visible light is absorbed by the molecule. Absorption of the UV-visible radiations results in the excitation of the electrons from lower to higher energy levels. In organic molecules only certain functional groups (chromophores) that contain valence electrons of low excitation energy can absorb ultraviolet and visible radiation. C-Cyts represent an ideal target molecule for UV-visible spectroscopy because of the large absorption of heme groups. The strong UV-visible absorption bands of the heme originate from the π→π* transitions, providing information about the type of heme, the oxidation, and the spin state of the central iron ion. UV-visible spectroscopy allows in vivo measurements of biofilms under physiologically relevant conditions (Fig. 4D ). In order to detect all the cytochromes (OMCs and inner membrane cytochromes) along the biofilm thickness without any spatial distinction growing the EABs on a transparent electrode (indium tin oxide) is suggested. 78 Moreover, by combining different experimental set-ups is possible to obtain a UV-visible spectrum of the OMCs only confined in the proximity of the electrode surface.
What is FUV spectroscopy used for?
Moreover, FUV spectroscopy can be utilized for qualitative and quantitative analyses of various liquid and solid samples, because each molecule shows a characteristic FUV spectrum with strong absorption, and intensities and wavelengths of FUV bands are very sensitive to changes in concentration, temperature, pH, and so on [ 46–50].
How many glucose units are in amylopectin?
146 UV spectroscopy studies of dextrins and amylopectin suggest that the outer branches of amylopectin are composed of approximately 18 glucose units and that the inner branches consist of about 10 subunits.
What are the advantages of FUV spectroscopy?
The most fundamental advantage of FUV spectroscopy is that it contains unique information about the electronic transitions and structure of molecules. One can obtain knowledge about them that is not accessible by any other spectroscopy.
Why are C-cyts used in UV spectroscopy?
C-Cyts represent an ideal target molecule for UV-visible spectroscopy because of the large absorption of heme groups. The strong UV-visible absorption bands of the heme originate from the π→π* transitions, providing information about the type of heme, the oxidation, and the spin state of the central iron ion.
Applications of UV-Vis Spectroscopy Technique
The applications of UV-Vis spectroscopy are enormous. The following are the main fields in which UV-Vis spectroscopy is used:
Application Fields
In addition to the above applications, there are still some fields where UV-Vis spectroscopy is widely used.
What is the purpose of forensic ink examination?
The purpose of forensic ink examination is usually to determine whether the writing on a document has been altered by establishing whether the ink-line from one part of the text is the same as or different to that from another part. Although several ink types and technologies are now in use, the traditional ball-point pen is still responsible for a large proportion of the writing frequently encountered in forensic work. The composition of these inks is based on glycerols to give high viscosity, alcohols together with soluble dyes to provide colour, and resin to bind the ink to the paper. Both black ink, based on the dye methyl violet, and blue ink, based on Victoria (Basic) Blue, are in widespread use and have been extensively studied.
How does PCA work?
PCA facilitates quantification of the degree of variability among spectra by characterising a set of spectra in terms of a small number of principal components, based on criteria of un correlated differences within the set . Consequently, each spectrum in the set is described by a linear combination of these principal components with the admixture coefficients (here called loadings) for each providing a measure of the similarity or difference between any pair in the set.
What is the UV spectrum of black ink?
For black ball-point pen inks, the UV/vis spectrum is dominated by the strong absorption at 585–590 nm from methyl violet. The precise position and shape of this feature depends on the mixture of partially demethylated species within the methyl violet formulation used by a particular manufacturer. In addition, a distinctive black hue is imparted to the ink through the addition of dyes absorbing across the remainder of the visible spectrum and into the near-UV; for example, yellow-orange dyes that absorb in the violet-blue region. Typical spectra shown in Figure 1 illustrate how readily many inks may be discriminated when subject to this analysis. These spectra were acquired in transmission on a Genesys 10 spectrometer using quartz cuvettes and a path-length of 10 mm.
What is the purpose of forensic analysis?
One of the aims in the forensic analysis of physical evidence is to compare a questioned material from a crime scene with a specific reference, for example from a suspect, to determine whether the two samples are indistinguishable. The examination of coloured materials such as inks and fibres falls into this category and presents a significant challenge to the forensic analyst who must determine whether the particular shades exhibited by the pair of samples may be discriminated or not.
What is UV spectroscopy?
UV/visible spectroscopy plays a key role in the discrimination of colour in the forensic analysis of fibres and inks. The application of chemometrics, however, is vital in many cases to enhance such discrimination and to put it on a quantitative basis so providing objective justification for the conclusions of the analyst.
How to obtain UV spectra?
UV/vis spectra from single fibres are often obtained using a spectrometer, such as the J&M Analytik MSP800 system , attached to a suitable microscope. Such an instrument enables acquisition of spectra from a spot size as small as 2 mm by 2 mm over a full wavelength range from 190 nm to 900 nm, limited only by the microscope optics. The fibre is mounted between a quartz slide and cover-slip, and then examined in transmission. A study involving a large variety of blue polyester fibres has been reported, 2 which demonstrates the discriminating power of this technique. In this work 263 fibre samples from known sources had their UV/vis spectra acquired using a 6 mm by 60 mm spot size. The traditional method of comparison is to simply overlay spectra in pairs. To provide enhanced discrimination two new approaches were adopted: comparison of derivative spectra and including the UV as well as the visible spectral data in the analysis. Derivative spectra are a particularly appropriate tool where broad convoluted peaks are present in the original spectra as this approach is sensitive to inflections and subtle gradient variation. This work showed that both the derivative spectra and the absorption spectra must be indistinguishable for a match to be obtained between two fibres. In several cases, discrimination between different fibres was achieved principally on comparison of UV rather than visible light absorption.
Why is trace evidence important?
Trace evidence such as fibres, hair or glass fragments has particular forensic significance due to its ease of transfer and subsequent persistence, particularly on textile substrates. Notable cases in the UK where fibre evidence has been of crucial importance include the murder of Sarah Payne in 2001 and securing the conviction of Steve Wright for multiple murders in Suffolk in 2006. 1
How is aerosol color measured?
Aerosol sample color is measured by means of its UV–Vis reflectance spectrum. Indeed, sample color strongly depends on its diffuse reflection: an object irradiated by a light source disperses part of the incident radiation by diffuse reflection, which is subsequently collected by the eyes of an observer which in turn act as transducers, converting the light signal into appropriate electrical impulses for the brain. Ultimately, these impulses are integrated and processed by the latter, which generates the color perception for the observer ( Kremers et al., 2016 ). Therefore, color is an extremely complex and subjective entity, as it is not a specific feature of the object itself, but depends on many variables such as light source, optical behavior of the object, observer's eyes and brain, etc. Since 1931, the International Commission on Illumination (CIE) has released guidelines to standardize color perception, based on the definition of three elements: light source, observer, and colorimetric spaces. The latter are mathematical models capable to define the color of an object in a rigorous manner ( Ibraheem et al., 2012 ). One of the most used colorimetric space is the CIE L*a*b* (CIELAB) ( ISO-CIE 11664-4-2019 ), which uses three cartesian components to uniquely define color sample: L*, which indicates the CIELAB lightness in the range 0 (pure black) to 100 (pure white); a*, measuring the CIELAB redness-greenness coordinate; and b*, associated with the CIELAB yellowness-blueness coordinate. This colorimetric space can also be defined in polar coordinates, thus obtaining the CIE L*C ab *h ab ° space (CIELCH) ( ISO-CIE 11664-4-2019 ), wherein: L* indicates the CIELAB lightness; C ab * represents the CIELAB chroma, a measure of the color intensity, defining to what extent a given color shade is “contaminated” by gray; and h ab ° indicates the CIELAB hue angle, whose value is expressed in degrees and describes the color tone. In particular, the 0° angle represents the red color. Because of their easy interpretability, in this work the mathematical definition of the colors of the analyzed portion samples was carried out employing the CIELAB and CIELCH spaces, starting from the reflectance spectra obtained and using the Color 2.01 software (Perkin Elmer Ltd, United Kingdom). Standard Illuminant D65 was set up as a representation of solar light source, according to CIE ( ISO-CIE 11664-2-2020 ), and an observer angle of 10° was set up with the aim of simulating the average spectral response in human observers ( ISO-CIE 11664-1-2019 ). Average values and the standard deviations of the colorimetric parameters (L*, a*, b*, C ab *, and h ab °) were calculated for the blank filter (B) and the 19 samples (SN1,…SN19). The CIELAB average data were used for color visualization through the online tool Nix Color Sensor ( https://www.nixsensor.com/free-color-converter/ ).
What is diffuse reflectance spectroscopy?
UV–Vis Diffuse Reflectance Spectroscopy is a widely used, basic spectrophotometric technique for the analysis of powders and surfaces, requiring a negligible sample preparation ( Torrent and Barrón, 2008 ). It is based on the surface dispersion of a fraction of the UV–Vis incident radiation on it. A UV–Vis collimated light beam is directed with a certain angle onto the sample and, as a result, an ensemble of optical processes leads to radiation reflection by the sample surface on the whole overlying hemisphere. As a rule, the radiation reflected by a sample can be considered as the sum of two components: regular (or specular) and diffuse (or nondirectional) reflectance (e.g., Torrent and Barrón, 2008 ). Regular reflectance occurs when incident radiation hits an ideally smooth and planar surface (i.e. without roughness) of the sample, and it is then reflected at an angle equal to the angle of incidence (Fresnel law). Instead, diffuse reflectance is a combination of several optical phenomena, such as multiple reflections, scattering and refraction, which disperse the radiation at all of the angles of the hemisphere of origin of the incident radiation ( Blitz, 1998 ). Diffuse reflectance, which depends on the physico-chemical properties and color of the surface, is therefore the most informative component ( Sellitto et al., 2008 ).
What is the material and method section?
The Materials and Methods section is organized as follows#N#(a)#N#Subsection 2.1 describes the PM 10 samples used for the application and characterization of the UV–Vis DRS methodology reported in this work ;#N#(b)#N#Subsection 2.2 describes in detail the method used, paying particular attention to the instrumental configuration and analysis method of PM filters ( subsection 2.2.1 ), how it is possible to parameterize the colour of the analyzed filters ( subsection 2.2.2) and obtain semi-quantitative data of iron oxide minerals from sample reflectance spectra ( subsection 2.2.3 );#N#(c)#N#Subsection 2.3 presents the validation of the proposed methodology. After checking the main results of the proposed methodology ( subsection 2.3.1 ), chemometric methods are used to identify the PM filters that have been subjected to a Saharan dust transport event ( subsection 2.3.2.1 ), and the diagnosis obtained are assessed and confirmed by a residence time analysis of back-trajectory ensembles ( subsection 2.3.2.2 ).
What is mineral dust?
Mineral dust represents one of the main components of particulate matter (PM) in the Mediterranean area. The rapid identification of Saharan dust events in PM samples is desirable and required for several reasons, including their role in direct effect on climate by radiative forcing as well as their adverse effects on human health.
Where is PM 10 collected?
PM 10 samples analyzed in this work were collected at Sierra Nevada, a high altitude site (37.096 N, − 3.387 W, 2550 m a.s.l.) in Southern Spain, within the framework of the Spanish national project FRESA (Impact of dust-laden African air masses and of stratospheric air masses in the Iberian Peninsula. Role of the Atlas Mountains, Ref: CGL2015-70741-R). The sampling station is located in an area scarcely influenced by traffic and other anthropogenic sources, but strongly impacted by Saharan dust incursion events due to its proximity to North Africa ( Fig. 1 ).
Where is mineral dust transported?
Mineral dust uplifted into the troposphere by turbulence and wind in the North African desert is often transported across thousands of kilometers including the Mediterranean basin, the European continent sometimes up to the northernmost countries and/or the American continent, according to the season (e.g., Middleton and Goudie, 2002 ). Overall the Mediterranean basin owing to proximity and average circulation patterns, is directly and frequently affected by the so called Saharan dust incursions throughout the year with events whose intensity and frequency are object of extensive research ( Brattich et al., 2015a, 2015b; Cabello et al., 2016; Cuevas et al., 2017; Cusack et al., 2012; Israelevich et al., 2012; Riccio et al., 2009; Tositti et al., 2014 ). This occurrence is being reported as increasing in likely connection with global warming ( Middleton and Goudie, 2002; Soleimani et al., 2020 ). These events have often been linked with PM 10 exceedances in respect to EU air quality standards drawing attention on potential health hazards as well as on their correct management ( Diapouli et al., 2017; Krasnov et al., 2014; Matassoni et al., 2011; Nava et al., 2012; Querol et al., 2019 ). The current EU Air Quality standards for PM 10 are far less stringent than the WHO standards with a threshold of 20 μg/m 3 (WHO annual mean) vs. a 40 μg/m 3 for the former ( WHO, 2006 ). It is to note that the EU regulation allows to eliminate from the annual inventory of exceedances the natural events such as Saharan dust incursions thus improving the overall environmental performance for this parameter ( EEA, 2012 ).

How Does A Uv-Vis Spectrophotometer Work?
Uv-Vis Spectroscopy Analysis, Absorption Spectrum and Absorbance Units
- UV-Vis spectroscopy information may be presented as a graph of absorbance, optical density or transmittance as a function of wavelength. However, the information is more often presented as a graph of absorbance on the vertical y axis and wavelength on the horizontal xaxis. This graph is typically referred to as an absorption spectrum; an example is shown in Figure 4. Based on the …
Strengths and Limitations of Uv-Vis Spectroscopy
- No single technique is perfect and UV‑Vis spectroscopy is no exception. The technique does, however, have a few main strengths listed below that make it popular. 1. The technique isnon‑destructive, allowing the sample to be reused or proceed to further processing or analyses. 2. Measurements can be made quickly, allowing easy integration into exper...
What Is Uv-Vis Spectroscopy?
How Does Uv-Vis Spectroscopy Work?
The Purpose and Applications of Uv-Vis Spectroscopy
Advantages of Uv-Vis Spectroscopy
Disadvantages of Uv-Vis Spectroscopy
- The main disadvantage of Uv-Vis spectrometers is their challenging assembly, and it may take time to prepare using them. Ensure that the area where you’ll place the device is clear of any electronic noise, outside light, and other contaminants that could affect the measurements and readings of the spectrometer. A Uv-Vis spectrometer is sensitive to...
Uv-Vis Spectroscopy Limitations
Uv-Vis Spectroscopy Is The Future