
What are some uses of UV/Vis spectroscopy?
13.21.1.1: Some Uses of UV/Vis Spectroscopy
- Electronic transitions. Let's take as our first example the simple case of molecular hydrogen, H 2. ...
- Looking at UV-vis spectra. We have been talking in general terms about how molecules absorb UV and visible light - now let's look at some actual examples of data from ...
- Applications of UV spectroscopy in organic and biological chemistry
Which spectrophotometer is the best?
Spectrophotometer DNA is an analytical instrument that can be used to measure the nucleic acid of a substance. Other instruments in this category include the NIR spectrophotometer which is considered by some experts as the best spectrophotometer in the world today. This is because of its accuracy and features.
What is a colorimeter vs. spectrophotometer?
• Colorimetry uses fixed wavelengths, which are in the visible range only, but spectrophotometry can use wavelengths in a wider range (UV and IR also). • Colorimeter measures the absorbance of light, whereas the spectrophotometer measures the amount of light that passes through the sample.
What is a cuvette blanked in a spectrophotometer?
“A blank cuvette is used to calibrate the spectrophotometer readings: they document the baseline response of the environment-instrument-sample system. It is analogous to “zeroing” a scale before weighing. Running a blank allows you to document the influence of the particular instrument on your readings.”
What is UV-Vis spectrophotometry used for?
Ultraviolet-visible (UV-Vis) spectroscopy is a widely used technique in many areas of science ranging from bacterial culturing, drug identification and nucleic acid purity checks and quantitation, to quality control in the beverage industry and chemical research.
What is principle of UV spectrophotometer?
The Principle of UV-Visible Spectroscopy is based on the absorption of ultraviolet light or visible light by chemical compounds, which results in the production of distinct spectra. Spectroscopy is based on the interaction between light and matter.
What is the difference between a UV spectrophotometer and a VIS spectrophotometer?
There is no difference between UV and visible spectrophotometer because both names refer to the same analytical instrument.
What is UV-VIS spectrophotometer scanning?
Scanning UV-VIS spectrophotometers are capable of quickly exposing the sample to the entire (or a set) range of wavelengths, and obtaining the absorbance measurements at all wavelengths across that range.
What is the range of UV-Vis spectroscopy?
In UV-Vis spectroscopy, wavelength is usually expressed in nanometers (1 nm = 10-9 m). The UV range normally extends from 100 to 400 nm, with the visible range from approximately 400 to 800 nm.
What is the function of UV?
UV radiation is widely used in industrial processes and in medical and dental practices for a variety of purposes, such as killing bacteria, creating fluorescent effects, curing inks and resins, phototherapy and suntanning. Different UV wavelengths and intensities are used for different purposes.
What are the 3 types of spectrophotometry?
A Quick Look at Types of SpectrophotometersSingle Beam:Double beam:Split beam:
What are the two types of spectrophotometry?
Among the different types of spectrophotometry, there are two primary methods employed; absorption spectrophotometry, which is concerned with the absorption of radiation and specific spectra of light, and Ultraviolet-Visible Range spectrophotometry, which is concerned with the reflectance of specific spectra of a given ...
What are the two basic types of spectrophotometer?
There are generally two types of spectrophotometers: a single beam, and double beam. Single beam spectrophotometers use a single beam of light – visible or UV – which passes through a sample in a cuvette.
What is the basic principle of spectrophotometer?
Spectrophotometer Principle. The spectrophotometer is an instrument which measures the amount of light that a sample absorbs. The spectrophotometer works by passing a light beam through a sample to measure the light intensity of a sample.
What is the unit of absorbance?
absorbance units (Au)Absorbance is measured in absorbance units (Au), which relate to transmittance as seen in figure 1. For example, ~1.0Au is equal to 10% transmittance, ~2.0Au is equal to 1% transmittance, and so on in a logarithmic trend.
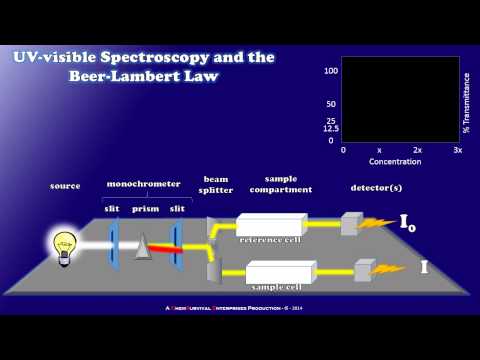
What are the main components of a UV-VIS spectrophotometer?
UV–visible spectrophotometers have five main components: the light source, monochromator, sample holder, detector, and interpreter.
When was the UV-Vis spectrophotometer invented?
It took more than 100 years until the first commercial UV-Vis spectrophotometer to qualify and quantify samples by the means of ultra violet and visible light was introduced by Arnold O Beckman in 1941 . The instrument utilized a quartz prism to separate light from a tungsten lamp into its absorption spectrum and a phototube, the predecessor of a modern photodiode to record the signal. To account for background influence from the lamp and the electronics a UV-Vis spectrophotometer measures the intensity of light transmitted through a sample and subtracts the described background automatically to provide precise readings that represent the determined properties of a sample.
What is UV Vis?
Ultraviolet-visible (UV-Vis) spectrophotometer is used to quantify and qualify samples by the means of UV and visible light (mainly 200 to 900 nm). The first mentioning of a spectroscope (predecessor of a spectrophotometer) dates back to 1814, when Joseph von Fraunhofer, the name patron of today’s world renowned Fraunhofer Gesellschaft, used his invention of this spectroscope to measure sunlight and discover the 574 dark fixed lines in the solar spectrum (Fraunhofer Lines). He also developed a diffraction grating in 1821 to separate the light from the sun, almost 40 years after the first manmade diffraction grating was invented by David Rittenhouse.
What is a nanophotometer used for?
The NanoPhotometer® is mainly used for nucleic acid (DNA, RNA, mRNA, Oligos with and without dye labels) and protein/antibody quantification and qualification, OD600 measurements and a lot of other applications like kinetics in a drop and scans of small molecules even in organic solvents. So let’s take a look at the specifications that are of relevance when planning your experiments (the specifications of the NanoPhotometer® are shown in parenthesis.)
What is the law of absorbing light?
The Beer-Lambert law, also known as Beer’s Law, empirically relates the absorption of light to the properties of the sample. This law states that there is a logarithmic relationship between the transmission of light through a specific sample (T = I/Io with I = outgoing light and Io = incoming light), the molar extinction coefficient for a specific compound (ε), the concentration of the absorbing species in the material (c) and the distance the light travels (d).
What is the web server for nanophotometer?
The built-in web application server is another highlight of the NanoPhotometer®. It allows to control the instrument and access data from any computer (Windows or Mac), tablet or phone (Android and iOS). The NanoPhotometer® can also be integrated in any LIMS via REST API.
Is a nanophotometer a monochromatic or polychromatic?
In comparison to the first commercially available UV/Vis spectrophotometer, which has been a monochromatic scanner, the NanoPhotometer® represents a new class of UV-Vis spectrophotometer instruments with a polychromatic rather than a monochromatic optical setup.
Does nanophotometer work with beer?
To run an experiment on the NanoPhotometer®, he only needs to swipe a small drop of beer and put it on the microvolume pedestal. Since daylight is considered white light (an overlay of the three basic colors red, green and blue – RGB) and beer is yellow, it can be expected that it absorbs light in the blue range and only lets pass green and red light (which when overlapped results in yellow to our eyes)… and voila: the scan confirms what we have anticipated perfectly!
What is UV-visible spectrophotometry?
UV–visible spectrophotometry is a well-established technique for the selective determination of Cr (VI) with good detection power. The standard method for the selective determination of Cr (VI) is based on the formation of a red–violet colored complex with 1,5-diphenylcarbazide under acidic conditions, which can be detected spectrophotometrically at 540 nm. In order to achieve good reproducibility, several conditions such as temperature or amount of reagent and acids must be kept strictly constant. The molar absorption coefficient is from 3.0×10 4 to 8.0×10 4, fairly high, allowing detection limits of ∼5 μg l −1 under optimized conditions. The complex is very stable (less than 2% signal reduction over 90 min) and only a few ions like Mo (VI), Cu (II), Mn (II), Fe (III), and Hg interfere, but only at high concentrations. Some of these interfering ions can be masked either with phosphate or ethylenediaminetetraacetic acid (EDTA) or kinetically differentiated. The main problem in its application is the acidic conditions needed for complex formation with the potential risk of reducing Cr (VI) especially in the presence of organic materials, normally present in samples like natural or waste water. In general, spectrophotometric determinations in sample solutions that are originally colored is problematical.
What is UV spectrophotometry used for?
Ultraviolet (UV)–visible spectrophotometry is widely used in biochemistry, both for the determination of species and for studying biochemical processes.
Why is UV spectrophotometry important?
UV–visible spectrophotometry is still important for LC and CE detection of numerous important naturally occurring groups of substances , such as flavonoids , phenolic acids , anthraquinones , and coumarins , because they have very characteristic UV spectra. NIR can also play an important role in phytoanalysis.
What is the UV/VIS method?
The ultraviolet/visible (UV/VIS) spectrophotometry was used for direct measurement of carbonate ions (CO 32 −) concentration. For example, CO 32 − absorbs light at wavelengths of less than ~ 250 nm, this facilities acidimetric titration with UV detection of most carbonate-containing natural waters and observe an increase in % transmittance [246]. Ariponnammal reported that CaCO 3 has three characterized wavelengths at 233.42, 254.91, and 356.52 nm [247]. Furthermore, Nangare described direct UV/VIS method for simultaneous determination of CaCO 3 and aspirin in tablet dosage form [248]. Fig. 20 represents the UV/VIS spectrum of CaCO 3 in 0.1 NaOH, recorded by a Shimadzu model 1700 double beam UV/VIS spectrophotometer, which shows a maximum at about 240 nm.
How to obtain IR spectra?
Just as with UV/visible spectrophotometry, it is possible to obtain IR spectra of microscopic pieces of evidence. Instruments can be obtained that have a microscope attached to the spectrophotometer. Through a light path, the source radiation is channeled through the microscope and the object and then to the detector. Both transmittance and reflectance spectra can be obtained. Some microscopes are outfitted with micro-reflectance objectives so that small amounts of opaque material can be analyzed directly. A large number of evidence types can be analyzed by IR microspectrophotometry. These include single fibers, paint chips including cross sections, drugs, inks, copier toners, polymers, and dyes and pigments. Figure 5.13 is an IR spectrophotometer with attached microscope.
How to determine total chromium?
In order to determine total chromium, Cr (III) has to be oxidized either during sample pretreatment or, more elegantly, online with Ce (IV) using a FI manifold. The methods of oxidizing chromium to the required state and of destroying the excess of oxidizing agent are of critical importance for the overall performance of the method. In the conventional analytical procedure an excess of permanganate has to be decomposed by reduction with azide or by precipitation as hydrous MnO 2, while an excess of peroxydisulfate can be decomposed by boiling the solution or by reduction with azide. Ce (IV) can be used for online oxidation; however, the blank introduced with this reagent seriously degrades the detection limit of this approach and the online method is less tolerant against interferents such as Fe (III), Mo (VI), Mn (II), or Cu (II). Other spectrophotometric methods based on complex formation with Cr (III) are less selective and sensitive and therefore are of not much importance.
Is spectrophotometry more sensitive than fluorescence?
Spectrophotometric methods are usually less sensitive than fluorimetric ones, which accommodate concentrations of a few nanomoles per milliliter; however, their range of applications are much broader, including inorganic species, organic compounds, proteins, etc., since UV–visible absorption is more universal a property than fluorescence. Spectrophotometry is especially useful for monitoring enzymatic reactions, either to determine the reaction products directly or to measure the reaction rate.
What is UV VIS Spectroscopy?
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. Ultraviolet-Visible (UV-VIS) Spectroscopy is an analytical method that can measure the analyte quantity depending on the amount of light received by the analyte.
What is UV spectrophotometer?
UV spectrophotometers measure the visible regions of ultraviolet light and can provide valuable information, as well as detect any impurities, about the levels of active ingredients present in pharmaceutical compounds.
What is UV Vis?
Ultraviolet-Visible (UV-VIS) Spectroscopy is an analytical method that can measure the analyte quantity depending on the amount of light received by the analyte. Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV / Vis) in the ultraviolet-visible spectral field refers to absorption spectroscopy ...
What wavelength is used to determine the concentration of an analyte?
In UV-Vis, a beam travels through a solution in a cuvette with a wavelength ranging between 180 and 1100 nm. The sample absorbs this UV or visible radiation in the cuvette.
What is the wavelength of UV spectroscopy?
The UV frequency is between 100 and 400 nm, and the visible spectrum is between 400 and 700 nm.
What is the principle of IR spectroscopy?
The principle of IR spectroscopy utilises the idea that molecules appear to absorb unique light frequencies that are typical of the molecules’ corresponding structure. The energies depend on the form of the molecular surfaces, the vibronic coupling associated with them and the mass corresponding to the atoms.
Where are UV visible spectrophotometers used?
UV-Visible Mid-range to Upper-end Spectrophotometers are typically used in research laboratories, including university and industrial laboratories.
